 Rabu, 8 Januari 2025 (08:53)
Rabu, 8 Januari 2025 (08:53)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
Balance the redox reaction by ion electron method or half reaction method. MnO4-+Br-=MnO2+BrO3-+OH- (My documentary) View |
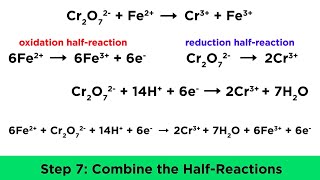 |
Balancing Redox Reactions in Acidic and Basic Conditions (Professor Dave Explains) View |
 |
MnO4^-+Br^-=MnO2+BrO3^- balance the chemical equation @mydocumentary838. mno4-+br-=mno2+bro3- (My documentary) View |
 |
Redox Balance MnO4- + Br- = MnO2 + BrO3- || Oxidation Number Method (Aurora Chemistry) View |
 |
Chapter 19:2 Balancing Redox Reactions-Basic (Nick Wright) View |
 |
Redox balance Br2 = BrO3- + Br- || Ion electron method || Half reaction method (Aurora Chemistry) View |
 |
MnO4^-+C2O4^2-=MnO2+CO3^2- balance the redox reaction by ion electron method or half reaction method (My documentary) View |
 |
balance by oxidation number methods MnO4- + Br- to give MnO2 + BrO3- in acidic Medium (Chembynlsir) View |
 |
MnO4-+I-=MnO2+IO3- balance the redox reaction by ion electron method in a basic medium. (My documentary) View |
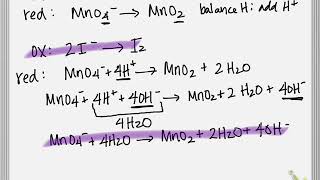 |
Balancing Redox Reactions (Basic Solution) Example (Professor Johnston) View |