 Selasa, 7 Januari 2025 (14:57)
Selasa, 7 Januari 2025 (14:57)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
An electron makes a transition from n = 2 level to n = 1 level in the Bohr model of a hydrogen atom. (cbse phyzics) View |
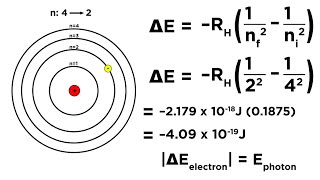 |
Bohr Model of the Hydrogen Atom (Professor Dave Explains) View |
 |
Q11 An electron makes a transition from n = 2 level to n = 1 level in the Bohr model of a hydrogen (physics spc sandeep sir) View |
 |
Electron Transition Bohr's Model 1 (Little Saigon #Wack) View |
 |
A hydrogen atom makes a transition from `n=2` to `n=1` and emits a photon. This photon strikes a (Doubtnut) View |
 |
First four Paschen series wavelengths from the Bohr atom energy level formula E n=(1/n^2)*E 1. (Zak's Lab) View |
 |
Week 12-4 Electronic Transitions in Hydrogen (Özhan Özatay) View |
 |
Bohr's Atomic Model 2 (Tierney Chemistry) View |
 |
Chapter 6: Explaining the Bohr Model and Energy Transitions (Carla Couch) View |
 |
The electron in a hydrogen atom at rest makes a transition from `n = 2` energy state (Doubtnut) View |