 Rabu, 8 Januari 2025 (12:59)
Rabu, 8 Januari 2025 (12:59)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
Fe2++Cr2O72-=Fe3++Cr3+ balance the redox reaction by ion electron method or half reaction method. (My documentary) View |
 |
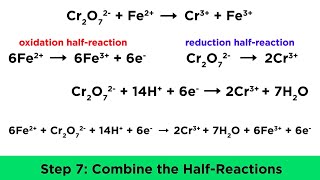
Balance the Redox Reaction for Cr2O7 2- + Fe2+ → Cr3+ + Fe3+ (Wayne Breslyn (Dr. B.)) View |
 |
Balancing Redox Reactions in Acidic and Basic Conditions (Professor Dave Explains) View |
 |
Balance Fe2+(aq) +Cr2O72-(aq) =Fe3+(aq) + Cr3+(aq) by ion electron method (Aurora Chemistry) View |
 |
Balancing Redox Reactions By Ion Electron Method | Easy Trick (Najam Academy) View |
 |
Half Reaction Method to Balance Redox Reactions (Najam Academy) View |
 |
Cr2O72-+SO2=Cr3++SO42- balance the redox reaction in ion electron method or half reaction method (My documentary) View |
 |
Balancing redox reaction in acidic medium by half cell method fe2++ cr2o72−→fe3++ cr3+ (NCERT chemistry) View |
 |
ion electron method || Vishal Rahal || redox reactions || balancing (The CheMaster) View |
 |
Cr2O7^2-+Fe^2++H^+=Cr^3++Fe^3++H2O balance the redox reaction in an acidic medium @mydocumentary838 (My documentary) View |