 Sabtu, 25 Januari 2025 (21:10)
Sabtu, 25 Januari 2025 (21:10)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
A central atom in a molecule has two lone pairs of electrons and forms three single bonds. The s... (PW Solutions) View |
 |
How to calculate bond pair and lone pair of electrons Easy Trick (Najam Academy) View |
 |
How To Draw Lewis Structures (The Organic Chemistry Tutor) View |
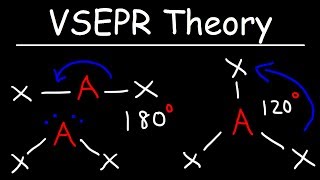 |
VSEPR Theory - Basic Introduction (The Organic Chemistry Tutor) View |
 |
Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures (ketzbook) View |
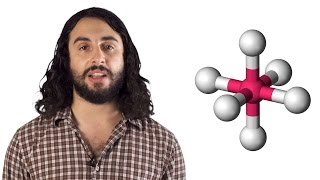 |
VSEPR Theory and Molecular Geometry (Professor Dave Explains) View |
 |
How To Identify The Number of Lone Pairs on an Atom Using Formal Charge (The Organic Chemistry Tutor) View |
 |
Trick to find lone pair of electrons and Hybridisation: Chemical bonding Tricks- NEET/JEE-NCERT (Chemistry Demystified) View |
 |
Hybridization of Atomic Orbitals - Sigma u0026 Pi Bonds - Sp Sp2 Sp3 (The Organic Chemistry Tutor) View |
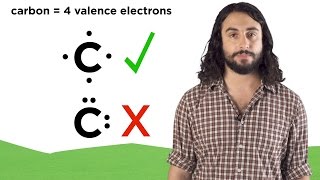 |
Lewis Dot Structures (Professor Dave Explains) View |