 Kamis, 23 Januari 2025 (23:33)
Kamis, 23 Januari 2025 (23:33)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
Calculate the concentration (in molarity) of a NaOH solution if 26.0 mL of the solution are needed (Not Your Chem Tutor) View |
 |
Solution Stoichiometry: Neutralization with Molar Concentration (Ms. Taylor's video tutorials \u0026 stuff) View |
 |
How many mL of 0.160 M HClO4 solution are needed to neutralize 33.5 mL of 0.0880 M NaOH (Not Your Chem Tutor) View |
 |
Solution Stoichiometry: Neutralization Reactions (Ms. Taylor's video tutorials \u0026 stuff) View |
 |
Ch 16 - Weak Acid Strong Base Titration Part 5 (addition of 26 mL of NaOH) (Hope Rindal) View |
 |
Titration calculations - Part 1 (Dr Leung's Science Channel) View |
 |
Molaritynotes42920 (Terry Koker) View |
 |
Ch2 4b (Dr. Perygin) View |
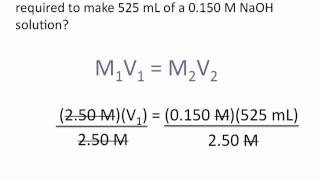 |
Dilution Problems - Chemistry Tutorial (TheChemistrySolution) View |
 |
WCLN - Concentration Problem - 1 - Chemistry (WCLN) View |