 Rabu, 16 April 2025 (20:33)
Rabu, 16 April 2025 (20:33)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
ch3nh3cl (OneClass) View |
![Download Lagu 14.82 | What is [OH−] in a solution of 0.125 M CH3NH2 and 0.130 M CH3NH3Cl Thumbnail](https://img.youtube.com/vi/yucP9Go22Uw/mqdefault.jpg) |
14.82 | What is [OH−] in a solution of 0.125 M CH3NH2 and 0.130 M CH3NH3Cl (The Glaser Tutoring Company) View |
 |
Calculate the ratio of CH3NH2 to CH3NH3Cl concentration required to create a buffer with pH = 10.22 (Not Your Chem Tutor) View |
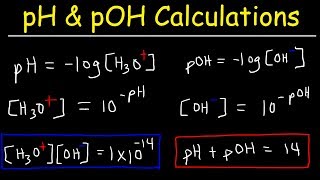 |
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems (The Organic Chemistry Tutor) View |
 |
ALEKS Problem solved - Calculating the pH of a buffer (Chemistry Stuff Explained!) View |
 |
Solve an equilibrium problem using an ICE table to calculate the pH of each solution a 0 18 M CH3N (HomewokLIB) View |
 |
Base Buffers and Equivalence Point (Niko Science) View |
 |
14.95 | The indicator dinitrophenol is an acid with a Ka of 1.1 × 10^−4. In a 1.0 × 10^−4-M solution (The Glaser Tutoring Company) View |
 |
14.90c | What is the pH of a solution that results when 3.00 mL of 0.034 M HCl is added to 0.200 L (The Glaser Tutoring Company) View |
 |
ch3nh3i (OneClass) View |