 Minggu, 23 Februari 2025 (03:17)
Minggu, 23 Februari 2025 (03:17)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
In a mixture of gases, the average number of degrees of freedom per molecule is 6 . The rms spee... (PW Solutions) View |
 |
The average number of degree of freedom per molecule for a gas is 7. A sample of the gas (Doubtnut) View |
 |
JEE Advanced Physics 2015 Paper 1 #16 (#2) Mixed Gases (Michel van Biezen) View |
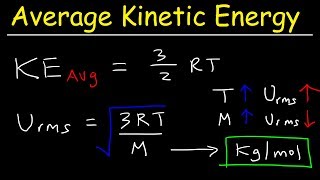 |
Average Kinetic Energy of a Gas and Root Mean Square Velocity Practice Problems - Chemistry Gas Laws (The Organic Chemistry Tutor) View |
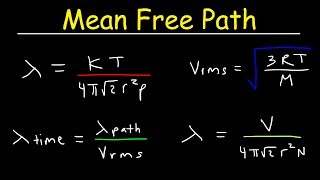 |
Mean Free Path, Mean Free Time, u0026 Root Mean Square Velocity Formula Chemistry u0026 Physics Problems (The Organic Chemistry Tutor) View |
 |
KTG|| Numerical on RMS speed (Physics Rahi) View |
 |
JEE ADV 2015 Q16 Paper 1 #iit #jeeadvance #booksolver #pyqs (Booksolver) View |
 |
The Ideal Gas Law and Kinetic Theory (Diana Driscoll) View |
 |
Question-90-Kinetic theory of gases (PHYSICS HUNT) View |
 |
(a) Calculate (i) root-mean-square speed and (ii) the mean energy of 1 mol of hyderogen at STP g... (Doubtnut) View |